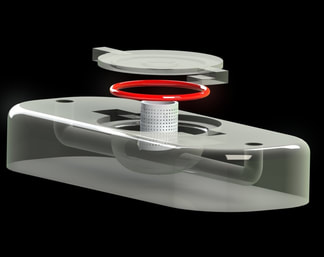
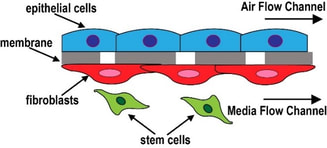
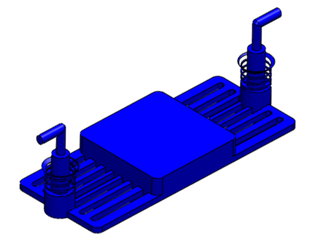
Research Overview
Spiraling costs of clinical trials are threatening the entire paradigm of drug discovery and development. There is a compelling need for creative screening approaches with higher predictive power for human level toxicity and efficacy. The Physiomimetic Microsystems Laboratory intends to spearhead a robust research program to develop long term human organotypic tissue culture and interrogation platforms. These platforms will have applications in the following distinct research areas: (i) A well validated drug testing platform for human diseases, (ii) fluidic perfusion and integration with similar platforms, (iii) simplistic models for pharmacokinetics and pharmacodynamic (PK/PD) studies, and (iv) stem cell culture and evaluation. These efforts will be highly interdisciplinary and will benefit from the participation of students, postdoctoral scholars, and faculty collaborators in biomedical engineering and the physical and clinical sciences.
Spiraling costs of clinical trials are threatening the entire paradigm of drug discovery and development. There is a compelling need for creative screening approaches with higher predictive power for human level toxicity and efficacy. The Physiomimetic Microsystems Laboratory intends to spearhead a robust research program to develop long term human organotypic tissue culture and interrogation platforms. These platforms will have applications in the following distinct research areas: (i) A well validated drug testing platform for human diseases, (ii) fluidic perfusion and integration with similar platforms, (iii) simplistic models for pharmacokinetics and pharmacodynamic (PK/PD) studies, and (iv) stem cell culture and evaluation. These efforts will be highly interdisciplinary and will benefit from the participation of students, postdoctoral scholars, and faculty collaborators in biomedical engineering and the physical and clinical sciences.
|
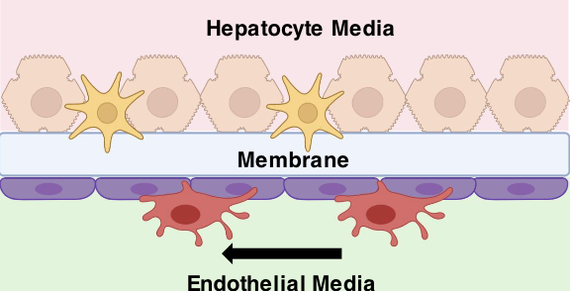
Liver on a Chip
Chronic viral hepatitis infection significantly increases patients’ likelihood of developing hepatocellular carcinoma (HCC). However, the mechanisms which govern the transition from viral hepatitis to HCC are poorly defined, and establishing chronic infection in vitro is extremely difficult, because primary hepatocytes do not typically maintain their mature differentiated state in traditional 2D culture for more than 2 weeks. We aim to develop a liver chip model that will provide hepatocytes with a more physiologically relevant environment, and prolong the functional lifespan of hepatocytes so they can sustain chronic viral hepatitis infection. We intend for this platform to: i) better maintain a mature hepatocyte phenotype, ii) sustain chronic viral hepatitis infection, and iii) allow for easy and rapid assaying of cancer markers to study the progression from infection to disease. [In collaboration with Dr. Emmanuel Thomas at Pathology, UM] |
|
Muscle on a Chip
The primary focus of the laboratory is to design, build, and test organomimetic cardiac, vascular, gut, and airway bronchial smooth ‘muscle-on-chip’ platforms. We will execute a research plan built around the three major challenges that the field of cardiovascular medicine is facing today: high attrition rates of drug candidates due to unforeseen cardiotoxicity, extremely slow progress of cardiac stem cell therapies, and stagnation in cardiac drug candidate development due to a lack of in vitro disease models. [In collaboration with Bhansali Lab at ECE, FIU] |
|
Cardiac Disease Models
Mechanical-stress induced hypertrophy and remodeling after myocardial infarction result in fibrosis, altered cytoskeletal architecture, and the increased incidence of cardiac arrhythmias. We intend to leverage our expertise in hydrogel design and engineering of the biotic/abiotic interface to create robust models of cardiac disease. Specifically, (i) by tailoring microenvironmental stiffness, we will study the arrhythmogenic potential of engineered tissues, (ii) we will initiate mechanistic studies into the mechanoelectrical signaling pathways of the human heart, and (iii) we will create models of reentrant arrhythmogenesis and cardiac amyloidosis. |
|
Pancreatic Islets on a Chip
A significant impediment to the development of cellular replacement therapies for Type 1 diabetes is the inability to sustain mature human beta cells in culture. We seek to engineer physiomimetic 3D niches within higher throughput microfluidics devices for maturation, maintenance and monitoring of human beta cells. The microfluidic devices will connect to universal docks and provide intimate control over the cellular microenvironment by independent modulation of liquid and gas phases, multiparameteric monitoring and assessment of cellular readouts and samplers for off-line biochemical analyses. We will then evaluate the effect of various niche parameters, such as the ECM, nutritional gradients, soluble factors, and oxygen microenvironments on human islet maintenance. [In collaboration with Stabler Lab at UF, and Ricordi Lab at UM] |
|
Lung Cancer on a Chip
Long term management of Lung Cancer remains a formidable therapeutic challenge. In a healthy individual, the volume of pleural fluid, an acellular liquid, is between 7 and 16 ml; however, lung cancer lead to an abnormal accumulation of fluid containing disseminated cells (up to 2 liters). Pleural effusions are valuable sources of diagnostic information as they can provide insight into patient health, such as the status of infections, inflammatory processes, and malignancy. We are designing, building, and testing an integrated microphysiological platform that would improve standard pleural effusion sample culture. Our approach is to mimic the complex cellular and extracellular matrix environments and microphysiological interactions that normally promote and maintain PE development. [In collaboration with Dr. Jorda and Dr. Gomez at Pathology, UM] |
|
Fibrosis on a Chip
Idiopathic pulmonary fibrosis (IPF) is an age-related, chronic lung disease with no cure. We are evaluating the regenerative capacity of bone-marrow derived mesenchymal stem cells (MSCs) within microfluidic cell culture platforms that mimic the ECM and cellular architecture of clinical IPF and allow high-resolution, real-time in-vitro analysis of biochemical, genetic, and metabolic activities. [In collaboration with Dr. Elliot and Dr. Glassberg at Pulmonology, UM] |
|
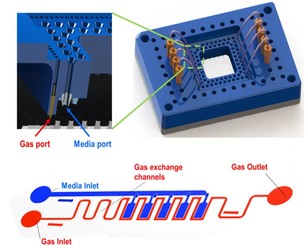
Microfluidic Device Design
A closely related effort in the laboratory is in developing novel and practical designs for microfluidic coupling of the organ mimics. It is critical for long term maintenance of organotypic cultures that they are connected to fluidic channels, pumps, pressure transducers, air bubble traps, valves and ports. We are designing control systems for microfluidic handling so that fluid flow can be stopped for optical interrogation of viability and function and for collection of samples for off line chemical analyses. These control systems will also permit optimal pre-conditioning of the organs before they are connected or interrogated. In addition, we are exploring housing, tubing, and adhesive materials which do not absorb small hydrophobic compounds and drugs. |